highlight all areas / label key areas

The production of energy in the form of adenosine triphosphate (ATP) is usually driven by the breakdown of fuels such as carbohydrate, fat or amino acids (from protein) via glycolytic (see Glycolysis pathways) and oxidative pathways (see The Citric Acid Cycle and Electron Transport Chain pathways). However, when the rate of energy demand is very high, the body utilizes the High Energy Phosphate Pathway (or Phosphagen system) for energy production.
The High Energy Phosphate pathways rely on the transfer of phosphate groups from ATP and its intermediates or from stored Phosphocreatine (PCr) to generate energy. These metabolic pathways are not primarily dependent on oxygen availability, nor do they rely on complex or multiple metabolic reactions. However, the concentration of ATP and PCr in cells is relatively low. As such, these pathways enable the generation of ATP to occur at extremely rapid rates but only for a relatively short time.
Adenosine triphosphate (ATP) is a high-energy phosphate and the primary energy source for all cellular processes in the body. ATP is used in all cellular homeostatic processes, such as making cell proteins, storing fuels, synthesizing RNA molecules, transporting substances into and out of cells and intracellular organelles, and aids in signaling to regulate cellular process. In addition, ATP plays a critical role in muscle contraction.
Despite the importance of ATP, the total amount of ATP stored within cells is actually very small, so the body relies on a number of metabolic pathways to re-synthesise ATP. The ability for ATP to rapidly turnover makes this a highly efficient molecule to meet the changing energy demands of the cell.
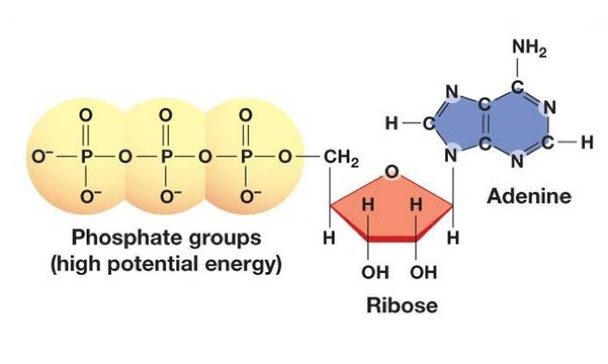
From a biochemical perspective, ATP is described as an adenine nucleotide, and consists of three components: a nitrogenous base (adenine), the sugar ribose and three phosphate groups. When ATP is broken down, the phosphate groups are removed, and in doing so, this releases a large amount of energy for use in cellular processes.
When the phosphate groups are removed, ATP is converted to either adenosine diphosphate (ADP; two phosphate groups) or to adenosine monophosphate (AMP; one phosphate group). Collectively, the sum of ATP, ADP and AMP is referred to as the total adenine nucleotide (TAN) content and remains relatively constant in cells under most conditions.
Key points:
Adenosine monophosphate (AMP) is an adenine nucleotide with only one phosphate group attached. AMP is an intermediate product of the adenylate kinase reaction.
AMP is a potent allosteric activator of the enzyme phosphorylase. This acts to increase glycogenolysis (see Glycogen Metabolism pathway) and subsequently glucose-6-phosphate, which is an important intermediate for glycolysis (see Glycolysis pathway). AMP also activates Phosphofructokinase (PFK), another important enzyme that increases Glycolysis (see Glycolysis pathway).
AMP can also play an important role in intracellular signaling. Changes in AMP levels can activate proteins such as AMP-activated protein kinase (AMPK) which can mediate gene transcription and cellular process associated with metabolism.
Note: AMP is not the same as cyclic AMP (cAMP). Hormones such as adrenaline or glucagon make cAMP from ATP using the enzyme adenylate cyclase.
Phosphocreatine (or creatine phosphate) is a high-energy phosphate (or phosphagen) that is capable of ATP re-synthesis at a very high rate.
Phosphocreatine transfers its phosphate group to adenosine diphosphate (ADP) to produce ATP and creatine, in a reversible reaction controlled by the enzyme creatine kinase.
ADP + PCr + H+ ↔ ATP + Creatine
The degradation of PCr is one of several pathways in the cell that is able to replenish ATP stores. However, the advantage of PCr is that it can supply ATP extremely rapidly, albeit for a short time, and is therefore able to buffer changes in ATP in the face of large fluctuations in energy demand.
The majority (92-96%) of phosphocreatine is found in skeletal muscle, with the remainder in cardiac muscle, brain and testes.
The amount of PCr stored in muscle (26 mmol/kg wet weight) is about 3-4 times that of ATP, enough to act as a temporary ATP buffer until other ATP re-synthesising processes such as glycolysis (see Glycolysis pathway) and oxidative (see The Citric Acid Cycle and Electron Transport Chain pathways) metabolism pathways reach maximal rates.
Adenosine diphosphate (ADP) is an adenine nucleotide with two phosphate groups attached. It is an intermediate product of ATP breakdown, but also an important substrate for ATP re-synthesis.
ADP levels remain relatively constant, except under conditions when the ATP synthesis rate is unable to match the rate of ATP breakdown (for example during high-intensity sprint type exercise).
Key points:
Inosine monophosphate (IMP) is an intermediate product of the Adenosine monophosphate (AMP) deaminase reaction.
An increase in IMP in the cell can be used as a marker of energy imbalance when ATP breakdown is greater than ATP re-synthesis.
IMP is phosphorylated so it is usually trapped in cells. However, some IMP can enter the purine nucleotide degradation pathway, which is a series of reactions producing inosine, hypoxanthine, xanthine and eventually uric acid.
Creatine kinase is the enzyme that controls the following reversible reaction:
ADP + PCr + H+ ↔ ATP + Creatine
The direction in which the reaction proceeds is dependent on the enzymes isoform.
For example, the muscle isoform (MM-CK) is found at sites of ATP utilisation (such as the myosin crossbridge heads) and drives the reaction in the forward direction to generate ATP. In contrast, the mitochondrial form of creatine kinase (Mt-CK) favours the reverse reaction that produces PCr.
Note: Skeletal muscle contains more creatine kinase than any other enzyme. Damage to the muscle cell membrane during exercise can result in creatine kinase leaking from the muscle into blood. Cardiac muscle also contains creatine kinase, so damage to the heart that can occur during ischemia or infarction (heart attack) can also result in the appearance of creatine kinase in blood.
Adenlyate kinase is an enzyme that results in ATP production (or re-synthesis) from Adenosine diphosphate (ADP). It acts to transfer a phosphate group from one of the ADP molecules to another and in doing so produces ATP and adenosine monophosphate (AMP).
ADP + ADP → ATP + AMP
This reaction will continue to produce ATP if there are adequate levels of ADP in the cell and if the free concentration of AMP remains low.
Key points:
Adenosine monophosphate deaminase (AMP deaminase) is an enzyme that converts adenosine monophosphate (AMP) to inosine monophosphate (IMP), and in doing so produces ammonia (NH3).
AMP → IMP + NH3
This reaction does not produce ATP, but is important as it ensures that the free-AMP concentration in a cell remains low. This also promotes phosphate transfer and ATP re-synthesis from ADP by minimising product inhibition of the adenylate kinase reaction.
Ammonia is the end-product of the Adenosine monophosphate (AMP) deaminase reaction. This can be toxic to cells but is usually removed into the blood for circulation to the liver and subsequently converted to urea through the Urea cycle (see Urea cycle pathway).
Adenosine Triphosphatase (ATPase) is a class of enzymes that removes the phosphate group (Pi) from ATP through a process of hydrolysis and in doing so releases energy for use in cell processes.
ATP + H2O→ ADP + Pi + energy
ATPases are usually described by their cell or tissue location and function.
For example, muscle requires an increase in energy for muscle contraction. For myosin cross-bridge recycling about 60-70% of the increased ATP breakdown occurs by myosin ATPase, whilst the remainder of ATP (20-30%) is used for sarcoplasmic reticulum calcium cycling by Ca2+ ATPase and in the muscle membrane (<10%) by Na+/K+ ATPase.