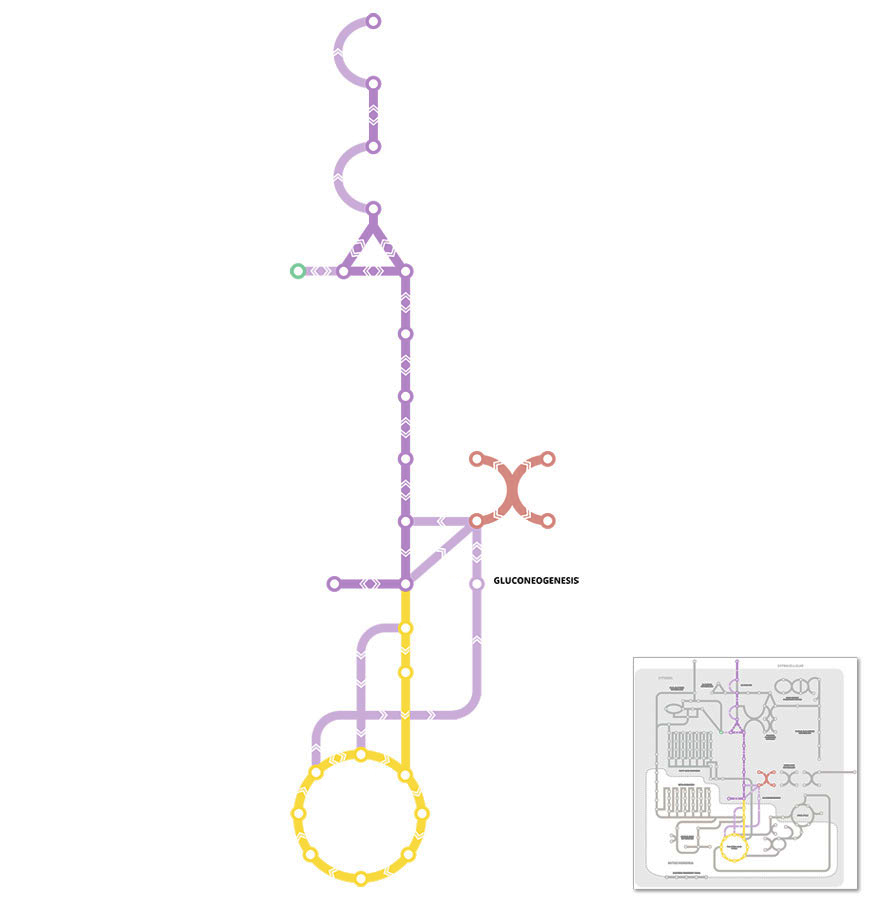
highlight all areas / label key areas

Gluconeogenesis is a metabolic pathway for the production of new glucose from non-carbohydrate precursors, such as lactate, pyruvate, glycerol and some amino acids.
Gluconeogenesis occurs mainly in the liver, and to small extent in the kidney. The process of gluconeogenesis becomes increasingly important when the liver is unable to produce enough glucose from the breakdown of liver glycogen stores via glycogenolysis (see Glycogen Metabolism pathway) to maintain blood glucose levels.
Conditions where liver glycogen levels are reduced or depleted and gluconeogenesis plays a more important role include after an overnight fast or starvation, when eating a low carbohydrate diet, and during the late stages of prolonged exercise.
For the most part, the process of gluconeogenesis is simply the reversal of glycolysis (See Glycolysis pathway) and relies on many of the same metabolic intermediates and enzymes that control reversible reactions. The primary difference is that 3 enzymes of glycolysis (glucokinase, phosphofructokinase, and pyruvate kinase) are replaced with Pyruvate carboxylase, Phosphoenolpyruvate carboxykinase (PEPCK), Fructose 1,6-bisphosphatase, and Glucose-6-phosphatase.
Key points:
Glucose (C6H12O6), a simple sugar, is a primary energy source for all cells/tissues in the body.
Endogenous glucose production occurs primarily in the liver, but also to a smaller extent in the kidney. Ultimately, the free glucose that is produced through these metabolic processes is exported from the liver via glucose transporter membrane proteins and released into the circulation to maintain blood glucose levels.
This enzyme removes the phosphate group from glucose-6-phosphate to create free glucose, as such this enzyme is found primarily in gluconeogenic tissues such as the liver.
Glucose-6-phosphate → Glucose + Pi
Only free glucose, the end-product of this reaction, can be released from the cell. This is the final rate-limiting step in gluconeogenesis and plays a key role in the regulation of blood glucose levels.
Key points:
Glucose-6-phosphate (G-6-P) is an important intermediate metabolite for endogenous glucose production.
In the liver, glucose-6-phosphate levels are produced in the cell either from gluconeogenesis, or through the breakdown of liver glycogen stores via glycogenolysis (see Glycogen Metabolism pathway).
The rate of gluconeogenesis is ultimately controlled by this key enzyme, which removes one of the phosphate groups from Fructose-1,6-bisphosphate and converts it to Fructose-6-phosphate
Fructose-1,6-bisphosphate → Fructose-6-phosphate + Pi
This is the reverse of the reaction that is catalysed by phosphofructokinase in glycolysis (see Glycolysis pathway)
Key points:
Glycerol is a key precursor for gluconeogenesis. Glycerol is released when triglycerides are broken down in a process known as lipolysis (see Fatty Acyl-Glycerol Metabolism pathway). This primarily occurs in adipose tissue, however free glycerol is released into the circulation by aquaporin transporters and it is then taken up by the liver.
In the liver, glycerol is first phosphorylated by the enzyme glycerol kinase to produce glycerol 3-phosphate, which then enters the gluconeogenesis pathway.
This enzyme regulates a key step of gluconeogenesis with the conversion of oxaloacetate to phosphoenolpyruvate.
Oxaloacetate + GTP → phosphoenolpyruvate + GDP + CO2
Key points:
Gluconeogenesis begins in the mitochondria and this enzyme regulates the first step or reaction where pyruvate is converted to oxaloacetate.
Pyruvate + ATP + HCO3 → Oxaloacetate + ADP + Pi
However, oxaloacetate cannot directly leave the mitochondria, so it must be first converted to malate via the enzyme malate dehydrogenase.
Key points:
There are two main forms of this enzyme, one found in the mitochondria and the other in the cytosol, and they are important because they control the reversible reaction between oxaloacetate and malate.
Oxaloacetate + NADH + H+ ↔ Malate + NAD+
The mitochondrial form of malate dehydrogenase, is a key enzyme of the citric acid cycle (see The Citric Acid Cycle pathway) that converts oxaloacetate to malate. This is important as oxaloacetate cannot exit the mitochondria so must first be converted to malate which can be transported out of the mitochondria.
Once malate has been transported out of the mitochondria into the cytosol, the cytosolic form of malate dehydrogenase converts malate back to oxaloacetate where it can then be eventually converted into glucose through the normal gluconeogenic pathway.
Amino acids become an important source of precursors for gluconeogenesis during the later stages of prolonged (>4 hours) exercise and under conditions of starvation. The loss of lean body mass seen during prolong starvation is a result of muscle protein being broken down and used for gluconeogenesis.
Amino acids firstly undergo a process of transamination or deamination that removes the amino group (see Amino Acid Metabolism pathway). This amino group is then converted mostly to urea in the urea cycle and excreted in the urine (See Urea Cycle pathway).
Following removal of the amine group the remaining amino acid carbon skeleton then enters into gluconeogenesis either directly (as pyruvate or oxaloacetate) or indirectly via intermediates of the citric acid cycle (see The Citric Acid Cycle pathway).
All of the common amino acids (except leucine and lysine) can have all or part of their carbon skeleton (termed an α-keto acid) used for glucose formation. Collectively they are called gluconeogenic amino acids.
Lactate is a major precursor for gluconeogenesis especially when blood lactate levels increase such as during hypoxia, or intense or prolonged moderate exercise.
For gluconeogenesis to occur using lactate as the precursor, lactate must first be extracted from the blood and taken up by the liver. In the liver it is converted to pyruvate via the reversible enzyme lactate dehydrogenase (see Glycolysis pathway). Pyruvate is then subsequently used to generate glucose via the process of gluconeogenesis for use by skeletal muscle and other tissues. This lactate-glucose cycle is call the Cori cycle.
This enzyme regulates the reaction where pyruvate is converted to oxaloacetate.
Pyruvate + ATP + HCO3 → Oxaloacetate + ADP + Pi