highlight all areas / label key areas

The electron transport chain is a series of reactions in the mitochondria that produce energy in the form of ATP.
This metabolic process utilises the NADH, and FADH2 (also termed intermediate electron carriers) generated by other metabolic pathways, including glycolysis (see Glycolysis pathway), beta-oxidation (see Beta-Oxidation pathway) and the citric acid cycle (see The Citric Acid Cycle pathway), along with oxygen, to synthesis ATP.
The oxidation of 1 NADH produces 3 ATP, whilst 1 FADH2 produces 2 ATP. Therefore, depending on the starting energy substrate the total number of ATP produced is:
For Glucose:
1 Glucose + 4H20 + 10NAD+ + 2FAD + 4(ADP + Pi) → 6CO2 + 4H+ + 10NADH + 2FADH2 + 4ATP
| Pathway | Yield | |
| Glycolysis | 2 ATP | |
| 2 NADH → | 6 ATP | |
| Pyruvate dehydrogenase reaction | 2 NADH → | 6 ATP |
| TCA cycle | 2 GTP/ATP → | 2 ATP |
| 6 NADH → | 18 ATP | |
| 2 FADH2 → | 4 ATP | |
| Total | 38 APT* |
For Fat (Palmitate):
1 Palmitoyl-CoA + 7CoA + 7H20 + 7NAD+ + 7FAD → 8 Acetyl-CoA + 7H+ + 7NADH + 7FADH2
| Pathway | Yield | |
| Beta-Oxidation → TCA cycle | 8 Acetyl-CoA → | 96 ATP |
| Beta-Oxidation | 7 NADH → | 21 ATP |
| 7 NADH → | 14 ATP | |
| Total | 131 APT* |
* Theoretically this is the total amount of ATP that can produced, however, the actual number of ATP available to the cell as an energy source will be less given that ATP is also used or consumed in these metabolic pathways. For example, ATP is used to transport molecules (such as NADH from glycolysis, see Glycolysis pathway) into the mitochondria, or to transport ATP out of the mitochondria after it is synthesised so it can be used by the cell.
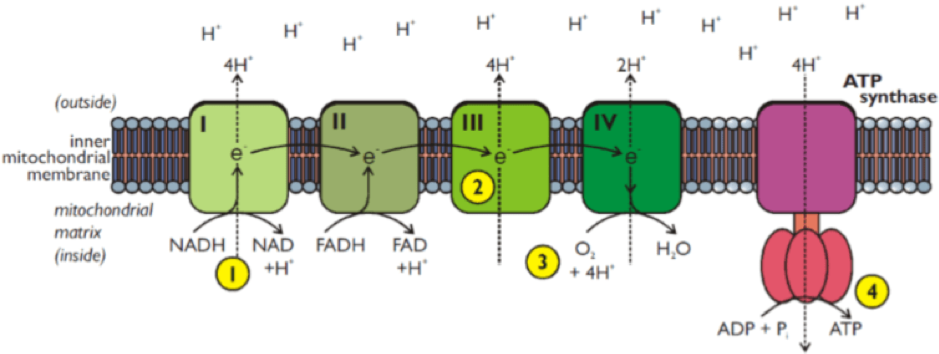
The electron transport chain uses NADH and FADH2 produced from other metabolic pathways in a series of reactions or protein complexes (Complex I-IV) in the mitochondrial membrane (See 1 in Figure).
These reactions produce NAD or FAD, hydrogen ions (H+) and electrons (e-). The electrons are transferred from one complex to the next until they are finally transferred to oxygen resulting in the production of water (see 3 in Figure).
As the electrons are passed from one complex to another, the hydrogen ions (H+) are transported across the inner mitochondrial membrane (see 2 in Figure), which establishes a concentration or electrochemical gradient (with a higher concentration of H+ in the intermembrane space).
Eventually the H+ flow down the concentration gradient and back into the mitochondrial matrix through an enzyme called ATP Synthase, and in doing so this provides the energy to synthesise or produce ATP (see 4 in Figure).