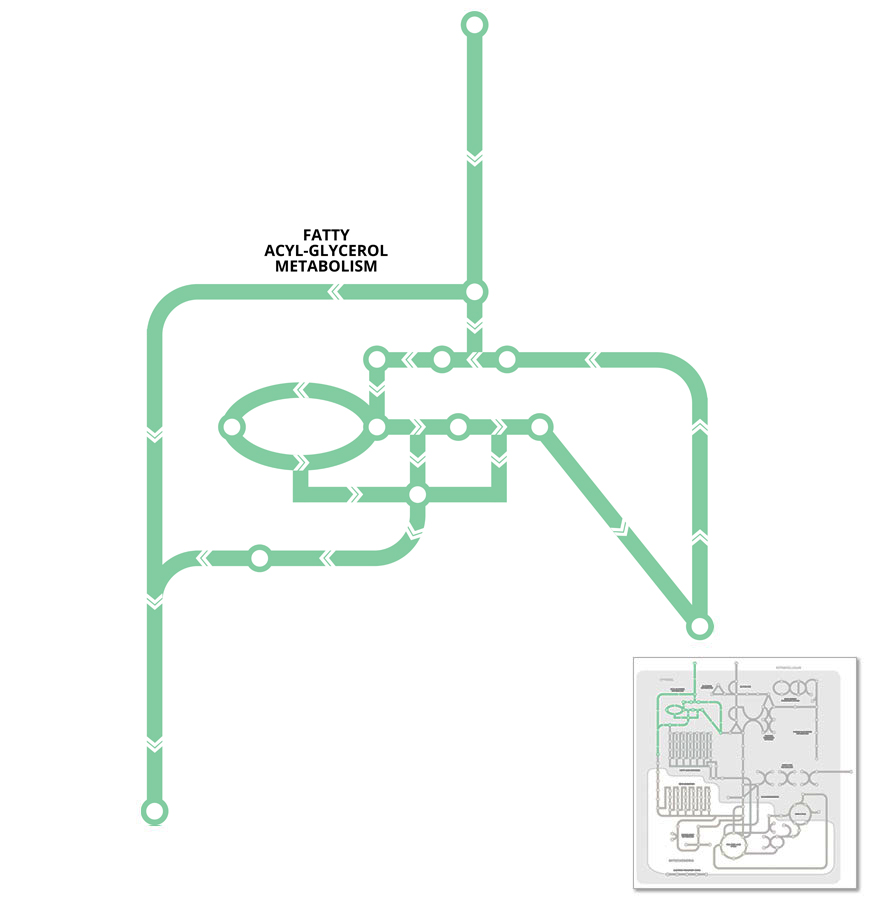
highlight all areas / label key areas

Fats or lipids are an important substrate for energy production, and are primarily stored in tissues as triglyceride. Triglycerides undergo constant recycling through pathways that promote triglyceride breakdown (lipolysis) to release fatty acids and glycerol, and re-esterification (or synthesis) of fatty acids and glycerol back to triglycerides.
For the most part, the process of triglyceride synthesis is simply the reversal of triglyceride lipolysis. However, to prevent futile cycling between the pathways different enzymes are involved at each step, so potentially both processes could be activated simultaneously but to a different extent.
Triglyceride synthesis or storage is usually favoured under conditions of nutrient excess when the energy demand is low. Whilst lipolysis occurs under conditions of increased energy demand such as muscle contraction or exercise, low calorie diets or fasting (starvation), or when the body is cold, in order to generate substrates that can be used for energy production (see Beta-Oxidation pathway).
Fatty acids are an important energy substrate for many cells/tissues in the body as they can be used in metabolic processes to yield large quantities of energy in the form of ATP. In addition to metabolism, fatty acids have important roles as integral structural components of cells by forming phospholipid membranes and in intracellular signaling. Fatty acids occur in many different forms and are usually categorised based on their structure or carbon-chain length.
Most of the fatty acids come from our diet where they are released into the circulation from the digestive system. Alternatively, circulating fatty acids are generated through the breakdown of stored triglycerides (lipolysis) usually from adipose tissues stores where they are transported to other tissues for use.
Free fatty acids have insoluble (or hydrophobic) components so when released into the circulation they are bound to transporter proteins such as albumin. In addition to lipolysis and fatty acid transport capacity, adipose tissue blood flow will also determine the mobilisation of free fatty acids into the circulation.
Key points:
Triglyceride (also known as triacylglycerol, TAG) is the stored form of fat, found primarily in adipose tissue stores under the skin (subcutaneous) and around internal organs (visceral). In a young healthy adult, adipose tissue stores average between about 20-30% of body weight, although this can vary greatly depending on age, gender, and genetic characteristics of the individual. These fat stores are enough to generate about 75,000 kcal of energy, compared to whole body glycogen stores that contain ~3000 kcal.
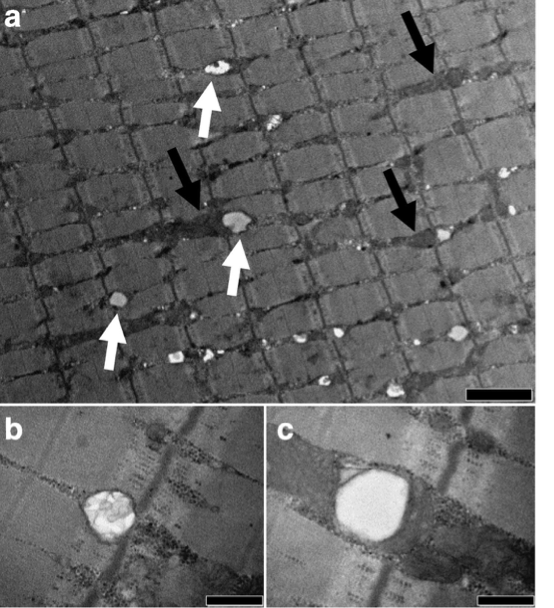
Other metabolically important, but smaller amounts of triglyceride can also be found in other tissues, in particular muscle (skeletal and cardiac muscle), referred to as intramuscular triglycerides (IMTG). Intramuscular triglycerides are located inside the muscle fiber or cell, and are stored in lipid droplets close to the mitochondria (see Figure below) where it serves as an important energy source for muscle contraction.
Triglyceride exists as a glycerol with three fatty acid chains attached. The amount of triglyceride is a balance between pathways that regulate triglyceride storage (synthesis) and breakdown (lipolysis).
Glycerol is the end-product of lipolysis (along with fatty acids). Glycerol can be recycled for de novo synthesis of triglyceride, but is also an important intermediate in carbohydrate metabolism. However, before glycerol can enter either of these pathways it must first be converted to glycerol-3-phosphate via the enzyme glycerol kinase.
In many tissues including muscle, the free fatty acids released during lipolysis are usually oxidised (see Beta-Oxidation pathway) to meet the energy needs of the cell. In contrast, in adipose tissue most of the free fatty acids from lipolysis are released into the circulation, where they are then available to be taken up by other cells/tissues.
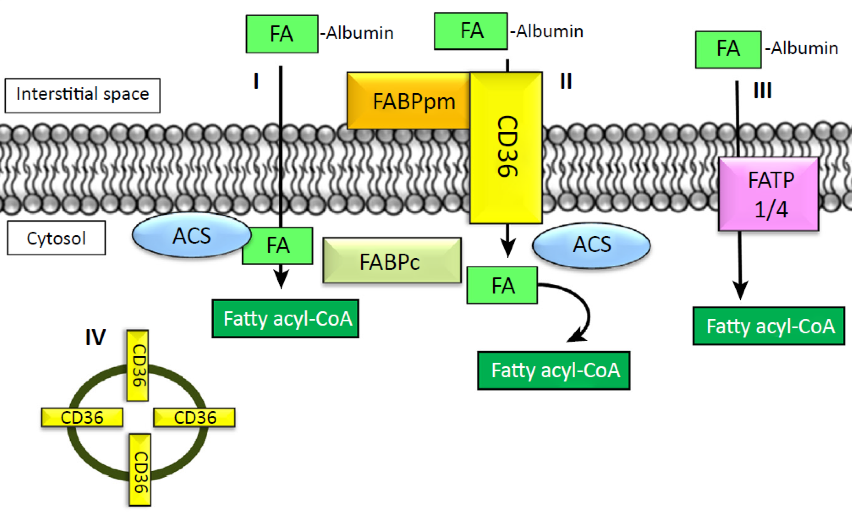
The transport of fatty acids from the circulation into cells, although not completely understood, can occur by both passive diffusion (I in Figure), and via facilitated transport by protein-mediated mechanisms at the plasma membrane (II and III in Figure) or by translocation to the plasma membrane from the cytosol (IV in Figure).
There are at least three distinct types of proteins that facilitate the uptake of fatty acids into the cell; Fatty Acid Translocase (FAT/CD36), Fatty Acid Binding Protein (FABP), and Fatty Acid Transport Protein (FATP).
CD36 can also interact with FABP at the plasma membrane (FABPpm) and cytosol (FABPc) which assist in fatty acid binding and facilitating transport to utilisation sites. These fatty acid transporter mechanisms are also associated with the enzyme Acyl Co synthestase (ACS) which act to trap the fatty acids with in the cell through the conversion to Fatty acyl CoA.
This enzyme initiates the first step of triglyceride synthesis, through the addition of one of the three fatty acyl groups to glycerol-3-phosphate.
Fatty acyl-CoA + Glycerol-3-phosphate → Lysophosphatidic acid + CoA
In subsequent reactions, additional fatty acids are added to produce intermediate lipid substrates that serve as critical components of biological membranes and mediate intracellular signal transduction. Along with Lysophosphatidic acid (LPA), these intermediate substrates include Phosphatidic acid (PA) and Diglyceride (or Diacylglycerol, DAG).
This enzyme regulates the last committed step in the synthesis of triglyceride or adipose tissue formation through the addition of the final fatty acid group to Diglyceride (or Diacylglycerol, DAG).
Diacylglycerol + Fatty acyl-CoA → Triglyceride + CoA
This enzyme removes the second fatty acid from triglyceride to produce monoacylglycerol.
Diacylglycerol → Monoacylglycerol + Fatty acid
As the name implies this enzyme is activated by enzymes such as adrenaline and glucagon that promote lipolysis. In contrast, HSL and subsequent lipolysis is inhibited by insulin.
This enzyme is the rate-limiting enzyme in trigylceride breakdown (or hydrolysis) as it specifically removes the first fatty acid from triglyceride to produce diacylglycerol.
Triglyceride → Diacylglycerol + Fatty acid
An increase in tissue levels of glycerol can activate the enzyme glycerol kinase to produce glycerol-3-phopshate.
Glycerol + ATP → Glycerol-3-Phosphate + ADP
Glycerol kinase is present mainly in the liver and kidneys, where the synthesis of glycerol-3-phophate depending on the physiological conditions can either enter the glycolytic pathway (see Glycolysis pathway) to provide energy for cellular metabolism, or be converted to glucose via the process of gluconeogenesis (see Gluconeogenesis pathway).
In muscle and adipose tissue, the amount of the enzyme glycerol kinase is much smaller, so most of the glycerol-3-phosphate for de novo triglyceride synthesis in these tissues is from the intermediate or by-product of glycolysis (see Glycolysis pathway). This also ensures that at least in adipose tissue most of the glycerol is released into the circulation for use by other tissues, and that some of the free fatty acids are available in the cell for oxidation (see Beta-Oxidation pathway).
Key points:
An important step for fatty acid uptake into cells is the conversion of fatty acids into fatty acyl-CoA. This process is controlled by the enzyme Acyl-CoA synthetase (ACS) through the following reaction:
Fatty acid + Coenzyme A + ATP ↔ Fatty acyl-CoA + AMP + PPi
This reaction ensures that the fatty acids are trapped within the cell to maintain a concentration gradient to allow fatty acids to continue to enter the cell.
Fatty acyl CoA is then available for further metabolic processes including oxidation (see Beta-Oxidation pathway) or converted into lipid and stored as triglyceride.
Key points:
This enzyme regulates the final step in lipolysis where monoacylglycerol is converted into fatty acid and glycerol.
Monoacylglycerol → Fatty acid + Glycerol